Which of the Following Describes an Endothermic Reaction Apex
The reaction mixture is sufficiently hot that the water is formed in the vapor phase. Hf products Hf reactants.
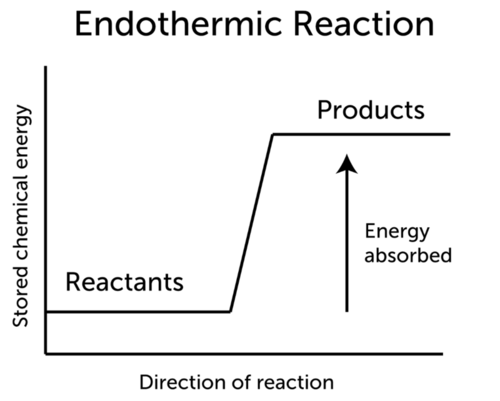
Endothermic Reaction Ck 12 Foundation
Wood burning in the fire heating its surroundings.

. An exothermic reaction is a reaction that releases energy to the environment. The piston moves as a result 50 kJ of work being. Option b Heating of water and Option d Melting of ice are View the full answer.
Which of the following statements best describes an endothermic reaction. This sentence is describing an endothermic reaction. To find out endothermic chemical reaction Ans.
Follow my ig flashcards containing study terms like What happens in a single-replacement reaction. Which of the following is described by the equation H2Os heat H2Ol. Part N Which following statements describes an endothermic reaction.
Thats for endothermic reactions actually. A B Heat -- C D. In the space below draw a.
The product of the reaction is water. Which of the following describes an endothermic change. How is an endothermic reaction identified.
Examples of exothermic changes. Thermal energy is absorbed by the system. It requires heat as a reactant.
In endothermic reactions energy is absorbed by a system from the surroundings. What is revealed in the reaction CaOH. Chemistry questions and answers.
Memorize flashcards and build a practice test to quiz yourself before your exam. And B is the activation energy in absence of enzyme which is lowered down to A in presence of enzyme. Chem 4-3 balancing nuclear reactions.
Option a Exposing water to cold temperature results in freezing and Option c metabolizing food are examples of exothermic processes. It is denoted by positive ΔH. The answer to your question is.
Example of a temperature change that might occur in an exothermic reaction. A reaction that converts thermal energy to chemical energy heat is taken in Endothermic reaction. Which of the following indicates an endothermic reaction.
Which of the following describes an endothermic reaction. Combustion neutralisation displacement condensation. Two reactants combine to form one product.
Chemistry chapter 5 Apex learning. How is the Hfusion used to calculate the mass of solid that 1kJ of energy will melt. Which of the following best describes a decomposition reaction apex February 11 2021 Uncategorized 0 Uncategorized 0.
Which of the following is exothermic. Which of the following describes an exothermic reaction A. Start studying the 424 Quiz types of reactions Apex answers chemistry a.
One reactant breaks apart to form new compounds. Sort the examples below into endothermic reactions and exothermic reactions. Drag the appropriate statements to their respective bins.
In endothermic reactions energy is absorbed by a system from the surroundings. Enthalpy is the heat involved in a reaction. Thermal energy is absorbed by.
Endothermic reactions have Positive Δ H. Cooking eggs photosynthesis burning a candle using an instant cold pack using a chemical hand-warmer car engine Endothermic reactions Exothermic reactions Cooking an egg Burning a candle Photosynthesis Hand warmer Cold pack Engine e. Energy is a reactant so energy is being taken in by the reactants to help them break their bonds.
Reset Help The energy level of the reactants is lower than that of the products The combustion of wood provides energy A reaction relatos 505 cal Exothermic Endothermic. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Thus in a chemical equation or reaction products will have more energy than the reactants.
Classify each of the following as an exothermic or endothermic reaction. Examples of endothermic changes. Libby N.

Which Of The Following Describes An Endothermic Reaction Brainly Com

Which Of The Following Involves An Endothermic Reaction Brainly Com

Which Statement Describes The Endothermic Reaction Represented By This Graph A Energy Is Absorbed Brainly Com

Which Of The Following Describes An Exothermic Reaction Brainly Com
Comments
Post a Comment