Formation of Dipeptide Bond
The ester bond was transferred to a peptide bond by incubation in a basic. It is possible to reverse this reaction by adding water.

Peptide Bond Definition Structure Mechanism And Examples
Polysaccharides may differ according to the type of monosaccharide they possess and the way the subunits bond together.
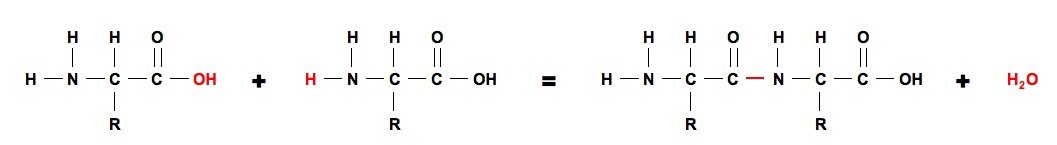
. A novel amide bond formation strategy from simple thioacid and amine starting materials is mediated by unstable but very reactive S-nitrosothioacid intermediates. This process continues giving long polypeptide chain of aminoacids. During the formation of this bond there is a release of water H 2 O.
To overcome these problems transition-metal-catalyzed site-selective Csp 3H functionalization has been developed with the assistance of various well-designed directing groups DGs that can coordinate to a metal center and deliver it on a targeted CH bond through an appropriate spatial arrangement enabling CH activation via the formation of a. The dipeptide on P-site is transferred to A-site forming tripeptide. This is because amino acid residues have an extra double bond.
NO O 2 has valence e_ in porbital. Nucleotides form bonds between the pentose sugar and phosphate group to form long polynucleotide chains. The proposed triad of linked equilibria inspired by findings from Frydman and coworkers Kaganovich et al.
Dehydration synthesis is also called as condensation reaction because both are characterized by the condensation and formation of water from the large molecule. Bond strength µ Bond order removal of electron from antibonding MO increases BO. This linkage is found along a peptide or protein chain.
However breaking a peptide bond this way occurs at a much slower rate than the dehydration synthesis reaction. Formation of a Dipeptide. Interfere with aminoacyl translocation reactions prevent release of uncharged tRNA from donor site after peptide bond is formed Erytnromycin 31.
Peptide formation occurs between the amino acids in the P site and the A site in the presence of the enzyme peptidyl transferase. With further additions resulting in the formation of a polypeptide chain. The extracted features along with machine learning.
The methodology is fully compatible with DNA. Organocopper chemistry is the study of organocopper compounds describing their physical properties synthesis and reactions. A catalytic hydrogen transfer reaction using PdC HCONH 4 and the micelle-forming surfactant TPGS-750-M for hydrogenolysis of Cbz-protected amines and benzyl protected alcohols and hydrogenation of alkenes alkynes nitros nitriles halides and aldehydes of DNA-conjugated substrates is described.
There are altogether 2765 descriptors that can be calculated using FEPS. The peptidyl transferase reaction transfers the amino acid from the P site onto the amino acyl-tRNA in the A site. IFPs are defined by linked equilibria involving folding through intramolecular interactions binding to components of the quality control machinery through specific heterotypic interactions and phase separation through homotypic intermolecular interactions.
Acyl imidazoliums have been long recognized as highly reactive acyl transfer agents in the context of amide bond formation. The first organocopper compound the explosive copperI acetylide Cu 2 C 2. While there are still only 20 amino acids a protein can be any length.
The formation of a peptide bond requires energy in the form of adenosine triphosphate ATP. Examples of dehydration synthesis reactions are the conversion of monosaccharides to complex sugars production of proteins from amino acids conversion of fatty acids to complex fats and the. Although some attention has been given to the activation of acyl imidazoles with Brønsted acids these species can be orders of magnitude less reactive than the N-alkylated analogues as was noted by Jencks and Lapshin in their kinetic studies.
Peptide bond formation. They are reagents in organic chemistry. A peptide bond is basically an amide-type of covalent chemical bond.
Interfere with formation of initiation complexes for peptide chain synthesis 2. The aminoacid present in t-RNA of P-site ie Fmet is transferred to t-RNA of A-site forming peptide bond. Organocopper compounds is the chemistry of organometallic compounds containing a carbon to copper chemical bond.
Parallel growth of peptides at various positions on. This bond links two consecutive alpha-amino acids from C1 carbon number one of one alpha-amino acid and N2 nitrogen number two of another. Indicating that the transformation of isoacyl dipeptide facilitated the formation of a β-sheet structure in SV28.
FEPS Feature Extraction from Protein Sequence webserver a comprehensive web-based feature extraction tool computes the most common sequence-driven features that are incorporated in 7 feature groups giving rise to 48 feature extraction methods. This fast reaction under mild conditions should be useful in synthesis. Incorrect statement for Tyndall effect is -A The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
A dipeptide or two amino acids bonded. On activation we observed formation of a central GV- and a terminal VV-dipeptide. Instead a ribosome works with tRNAs and mRNA to translate the language created by codons into a series of amino acids.
The second tRNA now carries a dipeptide and the first tRNA is without an amino acid. B The diameter of the dispersed particles is much.
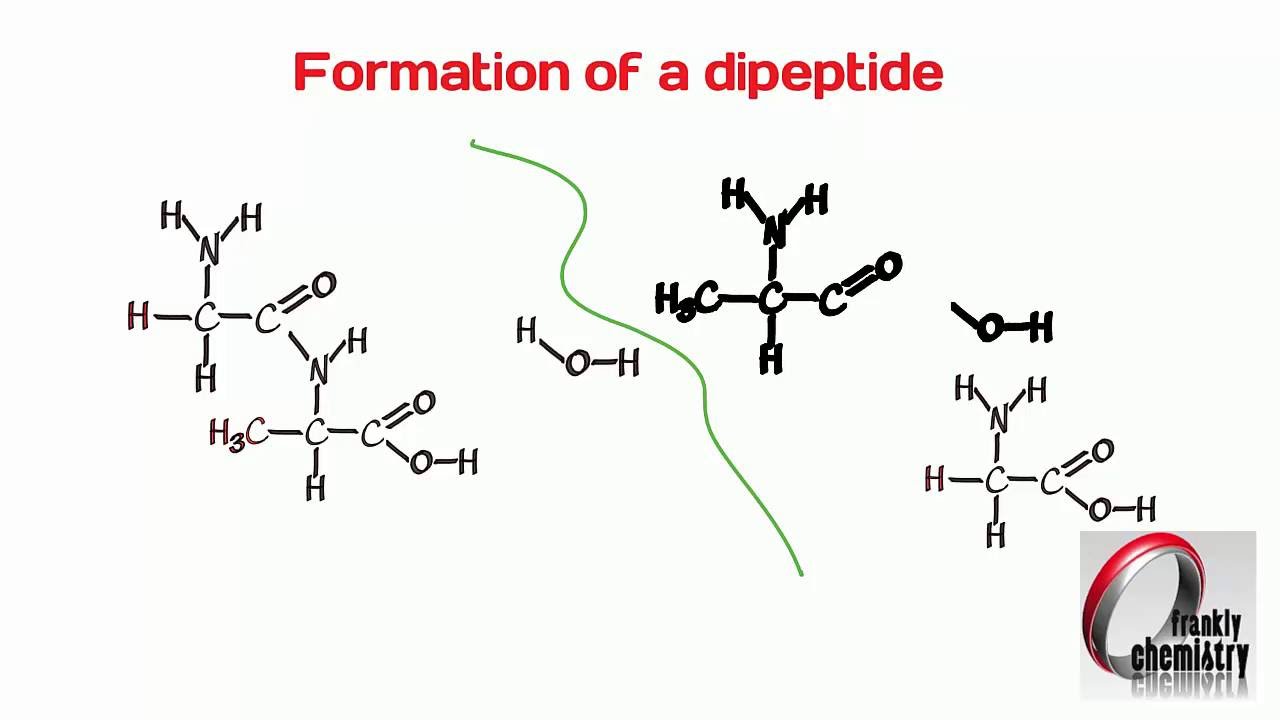
Amino Acids 4 Formation Of A Dipeptide Youtube
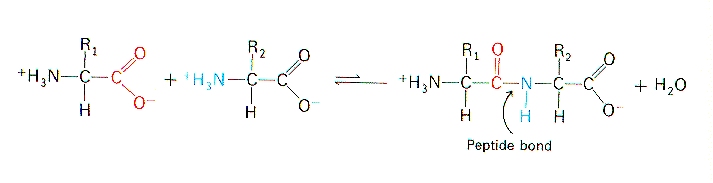
Peptide Bond The School Of Biomedical Sciences Wiki
Peptide Bond Formation Biochemistry Flashcards Draw It To Know It
Comments
Post a Comment