Barium Hydroxide + Hydrochloric Acid Balanced Equation
The balanced equation for this reaction is. The answer will appear below.
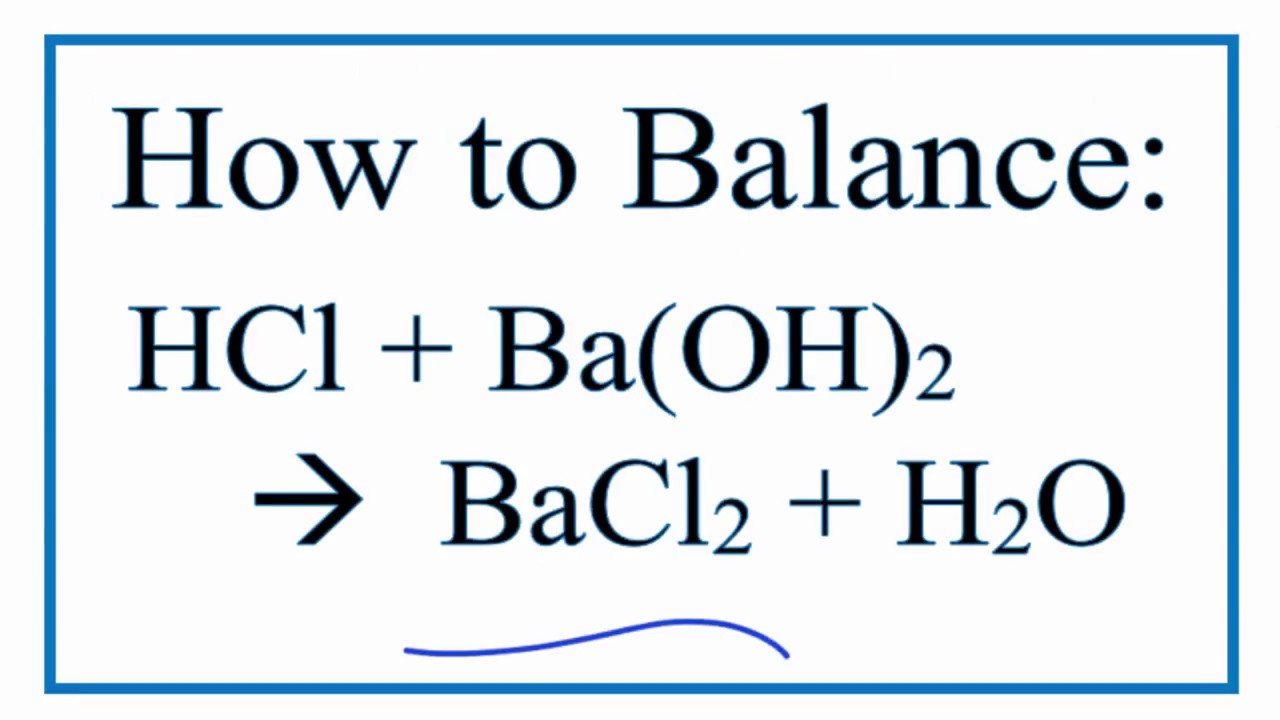
How To Balance Hcl Ba Oh 2 Bacl2 H2o Hydrochloric Acid Plus Barium Hydroxide Youtube
2 HCl aq Ba OH2 aq BaCl2 aq 2 H20 1 We can interpret this to mean.

. The balanced equation for this reaction is. BaCl2 2H2O What is the equation of barium sulphate with hydrochloric acid. 2HCl aq BaOH2 aq BaCl2 aq 2H2O l If 4 moles of barium hydroxide reactThe reaction consumes ____ moles of.
This forms a salt and water. Co - cobalt and CO - carbon monoxide. Fe Au Co Br C O N F.
2HCl BaOH2. The equation for this reaction is Ba OH2 2 HCl BaCl2 2 H2O. Balance the complete ionic equation when aqueous barium hydroxide reacts with aqueous hydrochloric acid asked Jun 26 2017 in Chemistry by Champoo ANSWER.
The reaction produces moles of barium chloride and moles of water. Let us verify if this equation is balanced or not. The balanced molecular equation for the reaction of hydrochloric acid and barium hydroxide follows.
2HCl aq Ba OH2 aq BaCl2 aq 2H2O l If 4 moles of barium hydroxide reactThe reaction consumes ____ moles of hydrochloric acid. 2HCl BaOH2. So Molarity of 37 HCl SOLUTION 370365 1014 M.
When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. It will give you 10 wv HCl.
Barium hydroxide is also a strong base strong electroyte so it also dissociates completely. 2HClaq BaOH2aq BaCl aq 2H O1 If 6 moles of hydrochloric acid react The reaction consumes moles of barium hydroxide. 2 HCl aq BaOH2 aq BaCl2 aq 2 H20 1 We can interpret this to mean.
Mole s of barium chloride and mole s of water. Ba HCl BaC l 2 H 2. 2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The.
This means for every mole of barium perchlorate produced 2 moles of perchloric acid and barium hydroxide are required. 2 moles of hydrochloric acid and moles of barium hydroxide React to produce. The resulting balanced equation is 2 HClO4 BaOH2 BaClO42 2 H2O.
So V 50 x 11014 493- ml. The balanced equation for this reaction is. Number of H atoms on LHS 4 RHS.
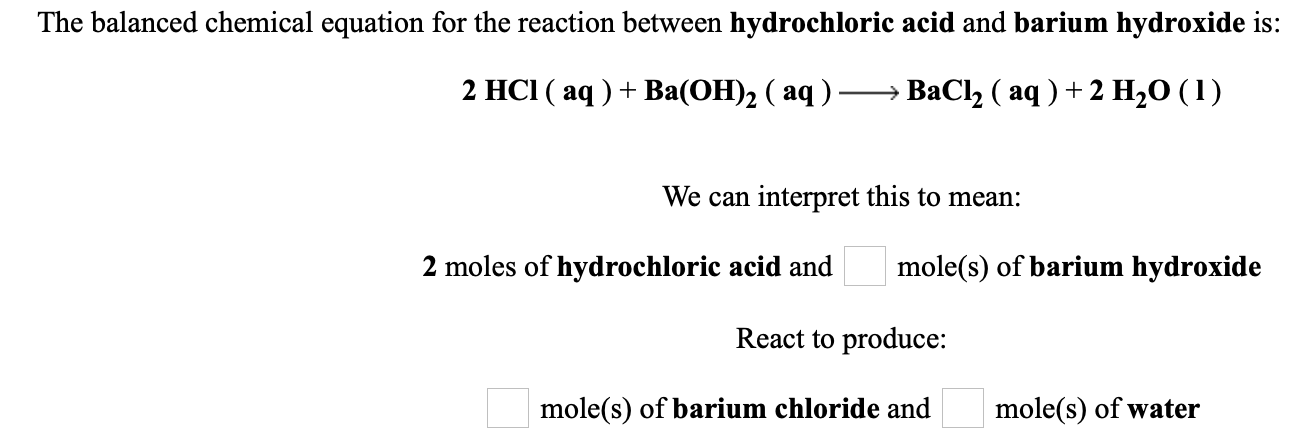
So 50 x 1 1014 x V. The balanced chemical equation for the reaction between hydrochloric acid and barium hydroxide is. By Stoichiometry of the reaction.
Number of chlorine atoms on LHS 2. When barium hydroxide is added to hydrochloric acid a chemical reaction takes place producing barium chloride and water. What is the balanced equation for the reaction of hydrochloric acid and barium hydroxide.
2HCl aq Ba OH2 aq BaCl2 aq 2H2 0 1 If 4 moles of barium hydroxide react The. The balanced equation for this reaction is. What is the balanced equation for the reaction between hydrochloric acid and barium hydroxide.
When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. In a balanced equation the number of atoms of each element will remain equal. The balanced chemical equation for the reaction between hydrochloric acid and barium hydroxide is.
37 HCl means 37- g of HCl is present in 100- ml of the solution. Take 10 gm of HCl powder then add 100 mL water. The equation for this reaction is.
When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water. 2 moles of aqueous solution of hydrochloric acid reacts with 1 mole of aqueous solution of barium hydroxide to produce 1 mole of aqueous solution of barium chloride and 2 moles of liquid water. Barium chloride and sodium hydroxide balanced equation December 24 2020 in.
Enter an equation of a chemical reaction and click Balance. Here 1 mole of barium reacts with 2 moles of hydrochloric acid to produce 1 mole of barium chloride and 1 mole of hydrogen gas. Finally we need to balance the above chemical equation.
B aO H_2a qH C la q rightarrow 2 H_2 OlB a C l_2a q c Perchloric acid is a strong acid meaning it is a strong electrolyte so it dissociates completely when in an aqueous solution. To enter an electron into a chemical equation use - or e. While balancing a chemical equation we need to determine the ratio of reactants to products which allows for the total number of atoms of reactants to match the number of atoms of the products.
B a 2 H C l B a C l 2 H 2 When barium reacts with hydrochloric acid it will form barium chloride with the evolution of hydrogen gas. The balanced equation for this reaction is. The option giving the balanced chemical equation of the reaction is 2HClBaOH2 ----BaCl22H2O.
When barium hydroxide is titrated with hydrochloric acid two molecules of hydrochloric acid combine with one molecule of barium hydroxide to produce one molecule of barium chloride and two molecules of water. So 1000- ml of the solution will contain 370- g of HCl. Ba OH2 is a strong base while HCl is a strong acid.
Ba2aq 2OH-aq 2Haq 2Cl-aq 2H2Ol Ba2aq 2Cl-aq. When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced. When hydrochloric acid reacts with barium hydroxide barium chloride and water are produced.
2 moles of hydrochloric acid and mole s of barium hydroxide React to produce. What is the balanced equation for the reaction of hydrochloric acid and barium hydroxide. The reaction of perchloric acid and barium hydroxide yields to barium perchlorate BaClO42 and water H2O.
Always use the upper case for the first character in the element name and the lower case for the second character.

Solved When Hydrochloric Acid Reacts With Barium Hydroxide Chegg Com
Solved The Balanced Chemical Equation For The Reaction Chegg Com

Comments
Post a Comment